Last year the Ye group conducted an actual laboratory astrophysics experiment. Graduate students Brian Sawyer, Ben Stuhl, and Mark Yeo, research associate Dajun Wang, and Fellow Jun Ye fired cold hydroxyl (OH) radicals into a linear decelerator equipped with an array of highly charged electrodes and slowed the OH molecules to a standstill. These molecules were then loaded into a permanent magnetic trap where they became the stationary target for collision studies. Next, Sawyer and his colleagues aimed supersonic beams of either helium (He) atoms or deuterium molecules (D2) at the OH molecules. They then studied the resulting low-energy collisions, which took place at temperatures of 80–300 K.
With respect to the D2 collisions, they wanted to see if their findings would shed light on the action of OH masers (See JILA Light & Matter, Spring 2008). Some theorists posit that OH masers emit coherent radio-wavelength photons after being excited by collisions with H2 molecules at temperatures only a few tens of degrees K lower than those in the laboratory experiment.
For this reason, the researchers planned to use H2 supersonic beams in their collision studies. However, their turbo pumps didn’t work with H2. Luckily, D2 molecules are chemically similar to H2, and their collision behavior was expected to be very similar to that of H2. The D2 molecules also had the advantage of being as massive as the He atoms, so the pumps worked.
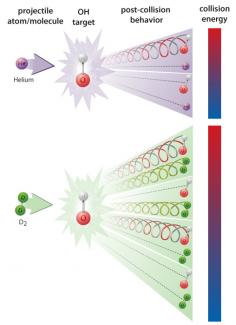
The results of the experiments depended on whether the molecular beam contained He atoms or D2 molecules. In a He–OH collision, the billiard ball-like He atom would either start an OH molecule spinning in an inelastic collision, or it would bounce off the OH molecule without rotating the molecule (elastic collision). When the collision energies fell below the rotational excitation energy in OH, the inelastic collisions stopped.
Things were more interesting when the incident beam consisted of D2 molecules. Here, the D2–OH collisional energies were the lowest ever measured in the laboratory. The much more complex D2 molecules were excited by some of the OH molecules via a collisional resonance that set both molecules spinning, as shown in the lower portion of the figure. Similar resonant energy transfer may also occur when H2 molecules collide with OH molecules in interstellar clouds where the average temperature is 30–50 K. The laboratory astrophysics project supported the idea that H2 molecules may be involved in stimulating OH masers. - Julie Phillips