The Ye group has built a cool new system for studying cold collisions between molecules. The system is far colder than a typical chemistry experiment that takes place at room temperature or hotter (300–500 K). But, it’s also much warmer than experiments that investigate ultracold-molecule collisions conducted at hundreds of billionths of a degree above absolute zero (0 K). The new system is known as “the cold molecule experiment” and operates at temperatures of approximately 5 K (-450 °F).
Now most people would still consider this temperature unbelievably cold. But for physicists, collisions between polar molecules at temperatures near 5 K are not just unbelievably cold. They’re also really interesting. That’s because cold collisions occur in the crossover region between the classical world familiar to most people and the mysterious quantum world.
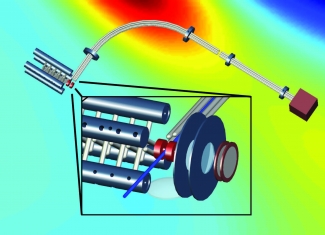
In this crossover region, the interactions of two molecules are simple enough to be modeled quantum mechanically, even though these interactions are far more complex than those between ultracold molecules at much lower temperatures. The new quantum mechanical description of cold collisions was provided by the Ye group’s theorist colleagues from Harvard and the University of Maryland. This work helped the experimentalists investigate the crossover region, where everything moves slowly enough to reveal new details about molecule collisions. The group’s investigations were made possible by the experimental setup shown in the figure.
This setup was designed by former graduate student Brian Sawyer, graduate students Ben Stuhl and Mark Yeo, research associates Matt Hummon and Yong Xia, Fellow Jun Ye, and colleagues from Harvard and the University of Maryland. The researchers used the apparatus shown in the inset to slow and trap a beam of hydroxyl molecules (OH) at a temperature of 70 mK. Then, they propelled a beam of ammonia made with heavy hydrogen atoms (ND3) mixed with helium (at 5 K) through the bent tube above the trap. A set of electrically charged rods guided only the ND3 around a bend and onto the trap holding the OH molecules.
Once the ND3 was flowing over the trapped OH, the experimentalists studied cold collisions between them. Because both OH and ND3 are dipolar molecules, they have an uneven internal distribution of electric charge resulting in one end being more positively charged, and the other end being more negatively charged. Cold dipoles exhibit interactions that are small compared to similar molecules at room temperature, infrequent, and, until now, hard to measure. However, with the new setup, the Ye group was able to observe these characteristically subtle effects and manipulate them by adjusting external electric fields. The researchers observed that external electric fields increased the number of collisions between the dipolar molecules that result in OH molecules disappearing from the trap.
Their theorist colleagues were able to explain that two different kinds of collisions were responsible for this loss. A few OH molecules were knocked out of the trap by “elastic” collisions. Such collisions occur when two dipoles fly past one another, and give each other a little kick. If the kick is hard enough, an OH molecule will fly right out of the trap. However, at temperatures of around 5 K, most elastic collisions aren’t hard enough to do this.
A second kind of collision, known as an inelastic collision, is actually responsible for most of the disappearance of OH molecules from the trap. This purely quantum mechanical interaction changes the quantum state of an OH molecule. Once this state change happens, the molecule simply falls out of the trap, since the trap only works on molecules in specific quantum states.
Clearly, there’s much more exciting physics to learn about the behavior of molecules inside the cold molecule experiment. That’s a good thing, too, because the cold molecule experiment is ideally suited for studying many of the molecules found on Earth and in space. -- Julie Phillips