Functional materials—like molecular electronics, biomaterials, light-emitting diodes, or new photovoltaic materials—gain their electronic or photonic properties from complex and multifaceted interactions occurring at the elementary scales of their atomic or molecular constituents. In addition, the ability to control the functions of these materials through external stimuli , e.g., in the form of strong optical excitations, enables new properties in the materials, making them appealing for new technological applications. However, a major obstacle to overcome is the combination of the very fast time (billionths of a second) scales and the very small spatial (nanometer) scales which define the many-body interactions of the elementary excitations in the material which define its function. The extremely high time and spatial resolutions needed have been extremely difficult to achieve simultaneously. Many physicists have, therefore, struggled to visualize the interactions within these materials. In a paper recently published in Nature Communications, JILA Fellow Markus Raschke and his team report on a new ultrafast imaging technique that could solve this issue.
A New Microscope for Quantum Interactions
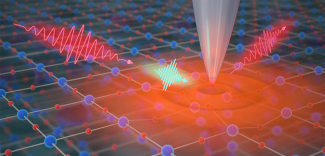
The technique is called ultrafast infrared pump-probe nano-imaging and builds on years of developments within the Raschke group. According to first author, and former postdoctoral researcher, Jun Nishida: “The technique we develop here is a new type of microscope. We have better, and now simultaneous, time resolution, spatial resolution, and also frequency resolution. We also made this very sensitive by combining different ways of modulating the laser light and how we detect it. This way we can selectively detect the tiny change in the already-small signal that comes from the nano volume [nanosized volume measurements]. With this development, we can study new phenomena that could not be probed with the previous existing microscope of this kind.”
The new ultrafast nano-imaging technique can probe functional material by exciting it with visible light and then imaging it in the infrared spectrum. “The materials’ electronic or optical functions arise from fairly low energy excitations and interactions within the atomic and molecular lattice,” explained Raschke. “That's why the infrared functional probing is really important. Whether it's a photovoltaic response or a quantum phase transition, many of the underlying many-body interactions all are low-energy excitations in the infrared energy range.” To achieve this goal, the team paired intense femtosecond laser pulses with a scanning probe microscope, where a tiny super-sharp metallic tip localizes the laser light resulting in an image with nanometer spatial resolution. By changing the timing between the laser pulses, they could take sequences of images and literally make movies of the motion of electrons and the coupled atomic lattice in the sample. Excited by their new method, the researchers knew the next step was to test it on a couple of functional materials to determine what useful information they would be able to get.
Rippling Electrons
The first functional material the team examined was vanadium oxide, a material that has intrigued physicists for decades. It can transition from an insulator into a metal by application of heat or light. “Vanadium oxide has potential applications for photochromic mirrors, for example,” Raschke said. “Imagine your window, transparent at low temperatures, becoming reflective at high temperatures. For example, a building may have entire windows made out of such types of material, where it will reflect later in the day when it gets hot, or converts the light into electricity, saving energy and money.”
“The material shows an insulator-to-metal phase transition with very complex interactions among electrons and atoms,” Nishida stated. “This is so complex that, even after half a century of intense debates, there are still ongoing discussions on the exact mechanism of the transition.” Raschke’s previous nano-imaging of nano-wires of vanadium dioxide revealed a high degree of disorder and heterogeneity (a material comprised of different ingredients) even in single crystals of the material, with regions within the wire more likely to transition to its metallic state than others. Because the material is made of differing ingredients, this difference can affect how the material transitions. “We really wanted to understand the nanoscale heterogeneity in this transition to have a full picture,” Nishida added.
The researchers used visible light excitation to induce the vanadium oxide to transition to metal, and then used infrared light to image the metallic state with their method. Raschke explained that their method mimicked ripples in a pond, as they perturbed the electrons in the material and monitored how it went back to its ground state. According to Nishida: “We used laser pulses to induce this transition, then image the transition with 100 femtoseconds (one millionth of one billionth of a second) and tens of nanometers (less than 1/1000 the thickness of a hair) resolution.” With their new technique, the team was able to witness how the heterogeneity affected the material's transition from an insulator to a metal. “Previously established mechanisms, such as strain, did not account for the heterogeneity we observed,” said Nishida.
Electron-Lattice Interactions in Solar Cells
The second material the researchers probed was lead halide perovskite. “This class of materials is a strong candidate for new and more efficient solar cells, which many people are looking into,” Nishida said. “It's competing with silicon because it's much cheaper. It's simpler to prepare and not as delicate, even if the surface quality is not very good, it still works very well.” Raschke explained the unique properties of the lead halide perovskite: “Light shines on the lead halide perovskite film, the electrons are excited by the light’s energy, and the electron is 'shielded' by the crystal lattice bending to stabilize the electron. So the electron is less likely to lose its energy before being converted to the desirable current the solar cell is supposed to produce.”
The researchers found that their new microscope can look into the intricate interactions between the excited electrons and a lattice. “Based on molecular vibrations of the lattice, we can look into the coupling between an electron and its surrounding lattice and molecules,” Nishida stated. “In this system, when a molecule ‘sees’ an electron in its vicinity, its vibrational frequency blue shifts. We find that this extent of the blue shift in the spectra of the material is different from point to point. This is really the first step to explain why perovskites are heterogeneous.” And adds Raschke, “Most importantly, it shows that perovskite solar cells have, by far, not yet reached their physical limit in terms of potential performance.” With their technique, the team of researchers was better able to understand how the interactions between electrons and atoms worked at the quantum level in a functional material, and therefore, the method can be used to guide materials science towards the targeted optimization of materials synthesis.
A Technique for Everything
Raschke, Nishida, Sam Johnson (a graduate student participating in the project), and their team are quite excited by their new technique. “Through the demonstration of the performance at [sic] these two different classes of materials, we were able to show how versatile our technique is, with its advances in multiple metrics of spatial resolution, time resolution, and sensitivity,” Johnson said. The researchers believe their ultrafast infrared pump-probe nano-imaging technique will be influential in studying more functional materials, leading to new discoveries in how they work.
Written by Kenna Castleberry, JILA Science Communicator