Brad Perkins and his thesis advisor Fellow David Nesbitt recently decided to explore what happens when fast, cold carbon dioxide molecules collide with the surface of an oily liquid (perfluoropolyether). Of course, you can only do these sorts of things in a vacuum chamber, where there are virtually no other gas molecules in the air to get in the way! The vacuum chamber itself creates an additional challenge: working with liquids at very low pressures.
For their experiment, the researchers used a supersonic expansion to create a molecular beam of carbon dioxide in hydrogen gas. They then measured the speed and temperature of the beam as it hit the surface of the liquid. Inside the vacuum chamber, the gas temperature was a cold 20 K, and, as it hit the liquid surface, the beam was traveling four times faster than the gas normally would at room temperature.
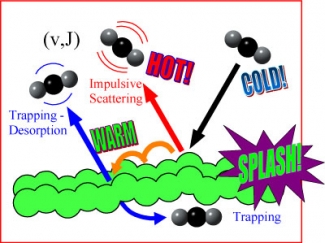
Under these conditions, about half of the carbon dioxide molecules splashed off the liquid surface as if it were a solid (impulsive scattering in the figure below); a second large fraction skipped across the liquid surface for a time before being reflected back into the vacuum (trapping desorption in the figure below). Initially, the trapping desorption behavior was like a carefully aimed rock that skips across the surface of a lake. However, the rock eventually sinks, unlike the carbon dioxide in this experiment. Only a tiny fraction of the original cold gas beam actually entered the liquid and stayed there (trapping in the figure).
Interestingly, the gas molecules that immediately splash off the surface come off at temperatures between 700 and 750 K, more than twice as hot as the temperature of the liquid, which is at room temperature (300 K). The gas molecules that stick to the surface as they skip along return to the vacuum at room temperature.
Perkins says that the molecules that splash off immediately get hot by transforming much of their energy of movement into heat. Because the sticky molecules dissipate some of their energy of movement as they skip over the surface, they only get warm. Perkins and Nesbitt reported this work in the July issue of the Journal of Physical Chemistry. - Julie Phillips