Imagine being able to study how molecules form on the quantum level. It turns out that researchers have already figured out some nifty techniques involving lasers and jets of reactive atoms for doing just that in a gaseous environment. Now graduate student Alex Zolot, former Visiting Fellow Paul Dagdikian of Johns Hopkins University, and Fellow David Nesbitt have taken this kind of study into a whole different arena: They recently probed the molecules that form when the surface of a liquid is bombarded with a very reactive gas.
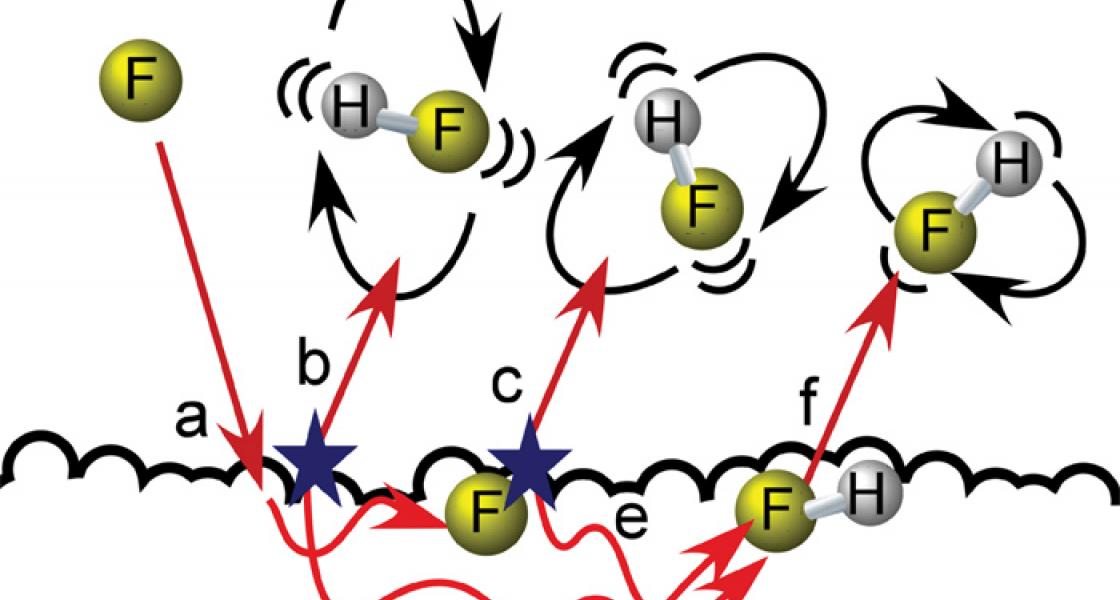
The liquid surface is an oily compound called squalane, a large natural molecule containing hydrogen (H) and carbon (C) atoms. It is purified from shark liver oil and sometimes used in cosmetics. The gas is fluorine (F), the most chemically reactive of all the elements. The resulting molecules are hydrogen fluoride (HF). They form when F atoms striking the liquid surface "pluck" a hydrogen atom from it and form a chemical bond. This particular chemical reaction releases lots of heat and energy. In fact, inside the vacuum chamber where the reaction takes place, there’s a small explosion happening!
After the molecules form and leave the liquid surface, Zolot and his colleagues examine them with an infrared laser beam aimed across the reaction chamber a little above the surface. The laser’s interactions with the molecules allow the researchers to determine not only the density of the molecules, but also any patterns in their velocities, vibrations, or rotations. This information, in turn, makes it possible to figure out how the molecules interact with the liquid surface after they are formed.
For instance, some new molecules fly off of the surface right away. They are vibrating intensely and spinning very fast (b in the figure above). They are also traveling very fast when probed by the laser beam. Their speed suggests they are likely very hot, in the range of 600–2800 °F. In other words, the fast-spinning molecules are also traveling very fast away from the surface.
The longer a molecule stays on the surface, the more its vibrations begin to calm down, the slower it moves, and the closer it gets to the temperature of the liquid (70 °F). The molecules still spin at a variety of speeds, as expected for HF at room temperature. However, the molecules continue to vibrate much longer. Only some of these interact with the surface so strongly that they stop vibrating; they come off the surface completely cooled to room temperature.
In a recent paper, Zolot and his colleagues explained these behaviors in detail. By elucidating the interaction of high-speed particles with surfaces, they hope to help other researchers and engineers better understand how high-speed atoms and molecules in space interact with satellites and come up with better strategies for preventing corrosion. In addition, their work may lead to a better understanding of atmospheric reactions at the surface of aerosol particles or oceans or industrial processes such as distillation, gas chromatography, and catalysis. - Julie Phillips