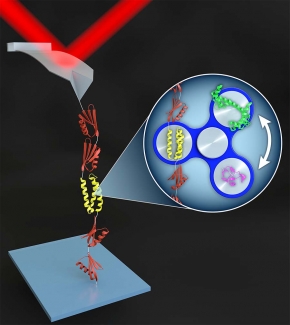
Over the last 50 years, the search to understand the protein-folding process has blossomed into a large, interdisciplinary field. More recently, mechanobiology—the study of the mechanical forces exerted on molecules and cells—has also become an important and rapidly growing field. In both fields, the energy landscape provides the framework to describe the process of protein folding and how proteins respond to force. Local and global minima on this landscape represent intermediate and final states, respectively. In single molecule force spectroscopy (SMFS), regions of the energy landscape away from the global minimum are probed by perturbing a biomolecule with applied force, just as global denaturants such as urea or temperature are used in traditional biochemical assays.
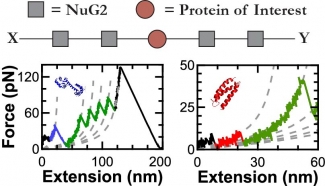
In one class of SMFS studies, an AFM tip pulls on a string of protein domains, typically with the protein of interest inserted as the middle domain. The resulting force-extension curves show a series of rapid force drops caused by the abrupt unfolding of the protein domains. Due to poor stability and force resolution, early AFM-based SMFS work was limited to proteins that unfolded at high force. Such “mechanically robust” proteins were generally fully or partially composed of β sheets. Studying the unfolding of α-helical proteins by AFM has been more challenging due to the lower forces involved.
In order to make measurements on the computationally designed alpha-helical protein α3D—the most mechanically labile protein yet studied by AFM—we integrated high-precision, low-drift cantilevers and site-specific coupling. Using these techniques, we measured its rate of unfolding, its folding as a function of force, and even stopped the cantilever altogether to measure its folding dynamics at equilibrium as a function of pH.
Now, we are extending our enhanced AFM techniques to a diverse array of systems. For example, two active projects are (i) studying the nanomechanics of human β-cardiac myosin’s catalytic domain and its lever-arm/light chain complex in collaboration Prof. Leslie Leinwand (CU) and Prof. Jim Spudich (Stanford) and (ii) determining the energetics and mechanical stability of tubulin within the microtubule lattice in collaboration with Dr. Antonina Roll-Mecak (NIH). We are always looking for new ways and collaborations to leverage these technological advances to provide enhanced biological insight.
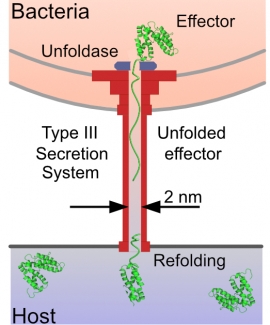
Beyond using applied force as a perturbing “denaturant,” there are also systems where mechanical unfolding of proteins is relevant in vivo! One example is the Type 3 Secretion System (T3SS), a syringe-like nanomachine that Gram-negative bacteria use to inject “effector” proteins directly into the cytosol of host cells, subverting their defense mechanisms and facilitating infection. At its narrowest point, the inside diameter of the T3SS’s needle is ~2 nm, so a protein must be unfolded to be secreted. Indeed, there are proteins (e.g., GFP) that will clog the needle. In a collaborative effort with Prof. Marcelo Sousa (CU), we characterized two effector proteins that readily pass through the needle. In contrast to prevailing theory, we found that the thermodynamic stability of two of these proteins was not atypically low and indeed similar to GFP and other proteins that clog the T3SS’s needle. However, in SMFS experiments, we found effector proteins to be among the most mechanically labile proteins ever characterized by AFM. Both the very low unfolding forces (<20 pN) and long distances to the unfolding transition state of these effector proteins aids their unraveling by the weak unfoldase of the T3SS. In identifying two effector proteins as thermodynamically stable but mechanically weak, this work thus provides a new paradigm for what makes effector proteins secretable.