Single-molecule studies are a powerful means to study the dynamics and energetics of biological molecules and biomolecular complexes. These studies are limited by instrumentation resolution and assay efficiency. Historically, AFM was considered to have force resolution of ~5–20 pN and to suffer from significant instrumental drift. Over the last 15 years, we have substantially improved the precision, stability, and temporal resolution of bioAFM, culminating in studying membrane-protein unfolding with a 100-fold improvement in time resolution and a 10-fold improvement in force precision.
Our first important development was to discover that the force stability of AFM is limited by the gold coating used to enhance cantilever reflectivity (and not, as expected, by tip-sample stability). By stripping off the gold, we decreased the drift rate by ~50-fold, achieving 0.5-pN force precision over a broad bandwidth (0.1–100 s). We next extended the timescale over which sub-pN precision was achieved to five orders of magnitude (0.001–100 s) by developing an efficient method to modify the shape of a commercial cantilever with a focused ion beam (FIB). This technique was then extended to ultrashort 9-µm-long cantilevers to achieve 1-µs resolution on a commercial AFM. These enhanced cantilevers enable us and others to study the folding/unfolding of proteins and other biomolecular interactions with a state-of-the-art combination of precision, temporal resolution, and force stability. We continue to develop more advanced cantilever designs that feature further improved performance.
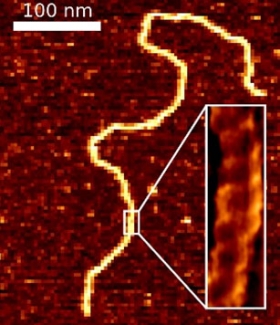
In a parallel effort, we recently overcame a 25-year-old problem in AFM imaging of equilibrated DNA in liquid: that DNA was not found to show the correct persistence length and height when deposited in biochemically relevant ionic conditions. We developed a simple 5-min protocol for depositing DNA and DNA-protein complexes onto mica in biologically relevant conditions (10 mM MgCl2, 25 mM KCl) that does yield the expected DNA structural parameters.
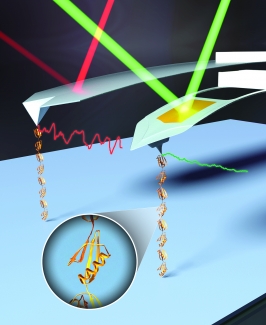
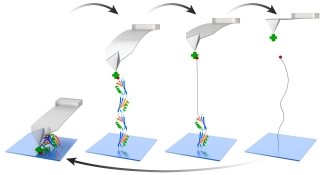
In SMFS, an important early advance was the head-to-tail linking of protein domains into polyproteins. The unfolding of polyproteins gives rise to a distinct mechanical fingerprint of an individual molecule, a sawtooth-like force-extension curve. The historical ease-of-use of AFM relied upon the nonspecific adhesion of polyproteins to a surface and a cantilever. However, such nonspecific assays result in a low yield of high-quality data, since adhesion can occur anywhere along the polyprotein. Additionally, nonspecific surface adhesion hinders studies of α-helical proteins, which unfold at low forces and low extensions. We overcame these limitations by merging two developments: (i) a polyprotein with versatile, genetically encoded short peptide tags for end functionalization and (ii) the efficient site-specific conjugation of biomolecules to PEG-coated surfaces. Heterobifunctional anchoring of this polyprotein construct and DNA via copper-free click chemistry to PEG-coated substrates and a strong but reversible streptavidin-biotin linkage to PEG-coated AFM tips enhanced data quality and throughput. For example, we achieved a 75-fold increase in the yield of high-quality data and repeatedly probed the same individual polyprotein to deduce its dynamic force spectrum in just 2 hours.
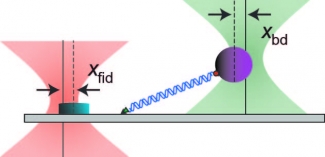
We have also made advances in the technology of optical-trapping SMFS experiments. The pioneering work to measure motion of a molecular motor at 1-base-pair resolution in the lab of Steve Block (Stanford) used a two-bead assay to decouple from the surface, helium to suppress noise, and a passive force clamp to eliminate the compliance correction. Using a series of active feedback loops, we eliminate all three of those requirements, enabling atomic-scale sensitivity to be brought to a much wider range of surface-coupled (single-bead) SMFS assays. We demonstrated 1 base-pair resolution by two different metrics, a step finding algorithm and the more traditional pair-wise distance distribution. Moreover, we achieved this resolution at a lower force (1.2–3-fold lower). In conjunction with this positional precision, we also improved the force precision of optical traps by actively suppressing laser intensity noise 100-fold. This increased instrumental precision is currently being applied to study a range of single molecules, especially helicases.