There are many molecules within the virus known as HIV, but only one is shaped like a hairpin. This molecule, aptly named the HIV RNA hairpin, allows the virus to create its own proteins from host resources, which is key to the virus’s ability to take over an infected cell.
Scientists having been studying the details of this virus for decades in an attempt to improve diagnostics and treatments for AIDS and other HIV-induced diseases. And now, JILA researchers have demonstrated a much easier, faster and more precise way to understand the structure and function of the HIV RNA molecule, especially the HIV RNA hairpin. Furthermore, the techniques developed for this research promise to allow a wider range of users to study similar biological molecules, as they are built upon commercially available and user-friendly atomic force microscopes, or AFMs.
AFM Advances
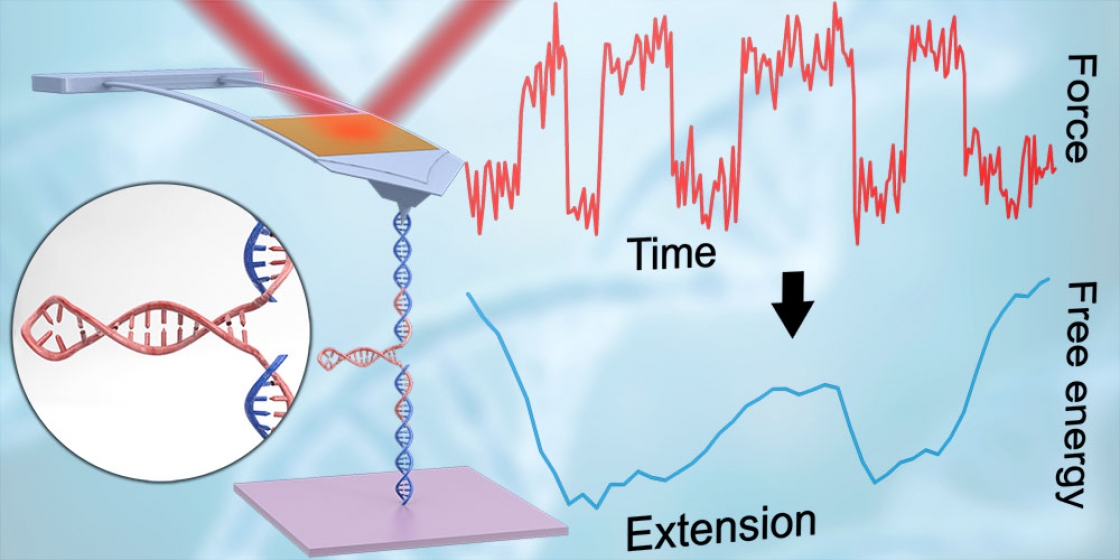
AFMs are high-resolution microscopes that can resolve structures as small as atoms. But on top of imaging, AFMs can also pull apart, or unfold, biological molecules such as proteins and nucleic acids like DNA and RNA.
Over the last decade, JILA Fellow Dr. Thomas Perkins and his team have focused on improving AFM technology for biological applications. Their advancements in cantilever shape provided enhanced time resolution and force precision. Their advancements in chemistry enabled individual molecules to be stretched end-to-end. And their advancements in instrument automation allowed researchers to unfold and refold the same individual molecule over a thousand times (whereas previously molecules could refold only a handful of times).
According to Perkins, his team’s advancements to AFM technology allow more researchers to probe biological molecules.
“It’s a question about accessibility,” said Perkins. “The benefit to doing these experiments on a commercial AFM is you can train an undergraduate to do the experiment.”
Energy Landscapes
Equipped with an advanced AFM, Perkins quickly pushed past old limitations of biological probes.
“We’ve spent all of this time overcoming various technical issues—cantilevers, surfaces, alignment—and now it’s this exciting time where we are doing applications, and we are doing things that people never expected you’d be able to do with an AFM,” said Perkins.
And one of the things never expected from AFMs was the ability to probe a folding molecule with the precision required to map out the energy landscape, said Perkins.
The energy landscape of a molecule is like a 3D topographic map whose peaks and valleys represent the energy of the molecule’s configuration. Because there are many ways a molecule can fold, there are many paths a molecule could take through this landscape.
And driving the molecule through this landscape is its desire to reduce its energy. Generally, a more folded molecule has a decreased energy. But some folding directions can be stymied by the equivalent of a high-mountain pass, or dam, in the landscape. Likewise, other foldings can be encouraged by sloping gullies. Understanding these complex landscapes—and how molecules navigate them—can help us understand why certain folds occur and why critical proteins may misfold and cause disease.
But diseases are caused by more than just misfolded proteins. Some diseases, like HIV, are caused in part by proteins made by an RNA hijacker called a hairpin.