Solvents — those things like water that dissolve other things like salt or sugar — are key players in some chemical reactions. That’s why the Lineberger group has come up with a nifty, but simplified, model system for studying solvent behavior. The group investigates the photodissociation and recombination of simple gas-phase anions, such as iodine bromide (IBr-), when they are surrounded by different numbers of carbon dioxide (CO2) solvent molecules.
Having everything in the gas phase makes it easier to probe chemical reactions than when everything is in a liquid state. Plus, gas-phase chemistry appears to closely mimic what happens in solution, as shown in a recent experimental/theoretical investigation by former graduate student Matt Thompson, graduate student Josh Martin, research associate Josh Darr, and Fellows Carl Lineberger and Robert Parson.
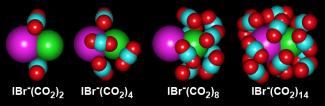
In this study, the researchers decided to figure out what had caused some very surprising experimental results about three years ago (See JILA Light & Matter, Summer 2006). In that work, researchers in the Lineberger group mixed gaseous streams of IBr- and CO2 to form a variety of cluster ions in which the IBr- was surrounded (solvated) with 1–16 CO2molecules. They used a mass spectrometer to separate out clusters having a particular number of solvent molecules. For each cluster size, they used a laser to break apart the IBr- molecules into I and Br- atoms. Without any solvent molecules around, the molecules stayed broken apart. However, as the number of CO2 molecules in the cluster increased, the fragments began to recombine to form IBr-. In fact, by the time there were 8 CO2 molecules surrounding each set of fragments, all the atoms recombined to form IBr-. Since the recombination efficiency was then 100%, the experimentalists figured that the reaction rate was likely very fast.
They were wrong. The recombination reaction rate as 1 to 4 CO2 molecules were sequentially clustered around each set of I and Br- fragments was about 10 ps. After that, the recombination rate slowed dramatically, becoming a 1000-fold slower (10 ns) by the time there were 8 CO2molecules per cluster.
To figure out what was going on, the Lineberger group consulted the Parson group. The Parson group simulated the cluster behaviors and found that when there were 8–10 CO2 molecules per cluster, all the CO2 molecules formed a solvent cage around the Br- that created a physical barrier to recombination. For recombination to occur, the solvent cage had to shift to over the I atom. Once this fluctuation happened, recombination occurred rapidly.
The reason recombination took so long was that it took a long time for the solvent cages to randomly fluctuate and move around the I atoms. Eventually they all did, and all the I and Br- fragments recombined. "What we saw was a version of the well-known mechanism of charge transfer in solution driven by the spontaneous fluctuation of a solvent cage," Parson said. The larger the solvent cage, the longer it took for this fluctuation to occur — up to a point. This point corresponded to a cluster size of about 10 CO2 molecules. Once the cluster size grew too big to fit around just the Br- fragment, it became easier for solvent-cage fluctuations to encompass the I atom.
The theorists predicted that when the fragment clusters consisted of more than 10 CO2 molecules, the recombination reaction rates would rapidly decrease. By the time the cluster size grew to 14 CO2 molecules, both photodissociated fragments would be fully surrounded by CO2 molecules, and recombination would again occur in about 10 ps.
When the Lineberger group recently tested these predictions in the laboratory, the trends in recombination reaction rates occurred as predicted. - Julie Phillips