Carl Lineberger and his group recently achieved some exciting firsts: (1) the experimental observation of the oxyallyl diradical, a key intermediate in a series of important chemical reactions, and (2) the posting of an abstract of the Angewandte Chemie cover story reporting this achievement — on Facebook! While the Lineberger group is responsible for the clever design of the photoelectron spectroscopy experiments that led to the observation of oxyallyl diradical, Lineberger was astonished that his work got on Facebook. He speculated that the journal’s publisher, Wiley-VCH, was responsible.
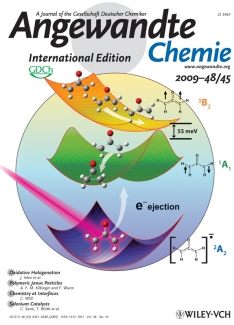
Wiley had also marketed the article with "refrigerator" magnets of the cover (shown here), reprints of the article, high-resolution copies of the cover art (designed by JILA’s own Greg Kuebler), wall calendars, and notebooks with the cover art on the front. A follow-up discussion of the story appeared weeks after the article was first published in October 2009. Welcome to the new world of marketing for research publications.
Wiley couldn’t have picked a more interesting topic for its marketing blitz. For more than 50 years, the oxyallyl diradical has been postulated to be a key intermediate step in important organic chemical reactions. However, it hadn’t been observed until the Lineberger group’s negative ion experiments supplied the first direct evidence for its existence. The group’s photoelectron spectroscopy studies also provided detailed information on the molecule’s electronic states and geometrical structure.
The secret of the group’s "transition state spectroscopy" is quite ingenious. Before attempting to observe the very unstable states of oxyallyl diradical, the researchers added an electron to the molecule, creating a stable negative ion. Then after carefully setting up their spectroscopy experiment, they ripped off the extra electron, then quickly looked for and found the molecule that chemists have predicted for so many years. Perhaps that’s why the Angewandte Chemie’s Notes page on Facebook announced the findings to the world.
These findings included the observation that the lowest-energy (singlet) state of the elusive oxyallyl diradical is a very short-lived transition state that rapidly rearranges to form a three-carbon ring called cyclopropanone. All that has to happen for the ring to form is for the two hydrogen atoms attached to oxyallyl diradical’s two end carbons to rotate away from each other in opposite directions (away from the plane of the molecule). The width of this feature in the spectrum indicates that the lifetime of the ground state is only about 30 femtoseconds. This very short lifetime is the reason that conventional attempts to generate oxyallyl had failed. The negative ion methodology was absolutely essential to detect the oxyallyl singlet diradical and characterize its rapid conversion to cyclopropanone. The group is now busy preparing a follow-on paper describing their new transition spectroscopy method in detail. - Julie Phillips