If you want to understand how chemical reactions happen, the ability to monitor dynamic positions of atoms in a molecule is critical. There's a well-known laser technique known as coherent Raman spectroscopy that uses a scattering laser pulse to set atoms vibrating and then measures the color shift of reflected light to detect vibration patterns. This technique has been used as a molecular fingerprinting device for simple motions of a molecule. However, if you want to probe rapid or complex three-dimensional atomic motion in molecules, current experimental techniques, such as electron or X-ray diffraction, are very limited in time or space resolution.
Now there's a creative (and much more sensitive) new experimental technique that combines aspects of Raman spectroscopy with electron diffraction that can be used to probe molecular dynamics. The technique comes compliments of Graduate Student Nicholas Wagner, Research Associate Andrea Wüest, Visiting Fellow Ivan Christov, Graduate Student Tenio Popmintchev, Graduate Student Xibin Zhou, and Fellows Margaret Murnane and Henry Kapteyn. The new method first uses an infrared laser pulse to excite a molecule, causing its atoms to vibrate. Then, in a process known as high-harmonic generation (HHG), a second higher-intensity pulse plucks high-speed electrons out of the vibrating atoms. As these high-speed electrons crash back into the molecule, they emit X-rays, whose strength depends on the position of the atoms in the molecule. The deBroglie wavelength of the high-speed electrons is close enough to the spacing between atoms to allow the researchers to "see" the atoms move back and forth when they observe the emitted X-rays using a spectrometer and a CCD camera.
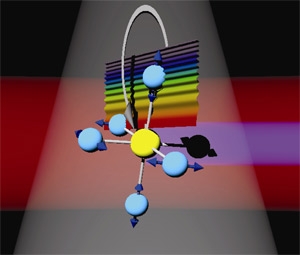
The researchers recently tried out their new method with the molecule sulfur hexafluoride (SF6), shown above. In the picture, the sulfur atom is yellow, and the fluorine atoms are blue (or black if one has temporarily lost an electron). The red band is the initial IR laser pulse, and the thin violet band comprises the soft X-rays emitted during HHG. The wavy rainbow represents an electron wave function in the process of being knocked far away from the molecule. The gray band and arrow indicate the wave function's return path to the molecule. In their experiment, the researchers were able to identify three distinct vibration modes of SF6; conventional Raman spectroscopy only revealed one of them, a particularly strong vibration in which the fluorine atoms move in and out from the sulfur atom in sync -like they're breathing.
Intrigued by their results, the researchers sought help from theorists Chris Greene, Greene's doctoral students Zach Walters and Stefano Tonzani, and Christov. Their analysis predicted that this kind of experiment should reveal up to six distinct vibrational modes, if a simple change is made to the wavelength range of the first laser pulse. Naturally, the experimentalists are busy refining their technique to find the modes they haven't seen yet and further demonstrate the potential of this new technique. - Julie Phillips
The work reported here appeared in the July 31, 2006, online issue of the Proceedings of the National Academy of Sciences.