The Lewandowski group recently decided to see what would happen if it could get cold molecules (1K–1mK) and ultracold (1mK) atoms to collide. Former graduate student L. Paul Parazzoli, graduate student Noah Fitch, and Fellow Heather Lewandowski devised a novel experiment to determine the collision behavior of cold (100 mK) deuterated ammonia (ND3) molecules and ultracold (600 microK) rubidium (Rb) atoms. The researchers hoped their experiment would help elucidate the role of quantum mechanics in molecular collisions.
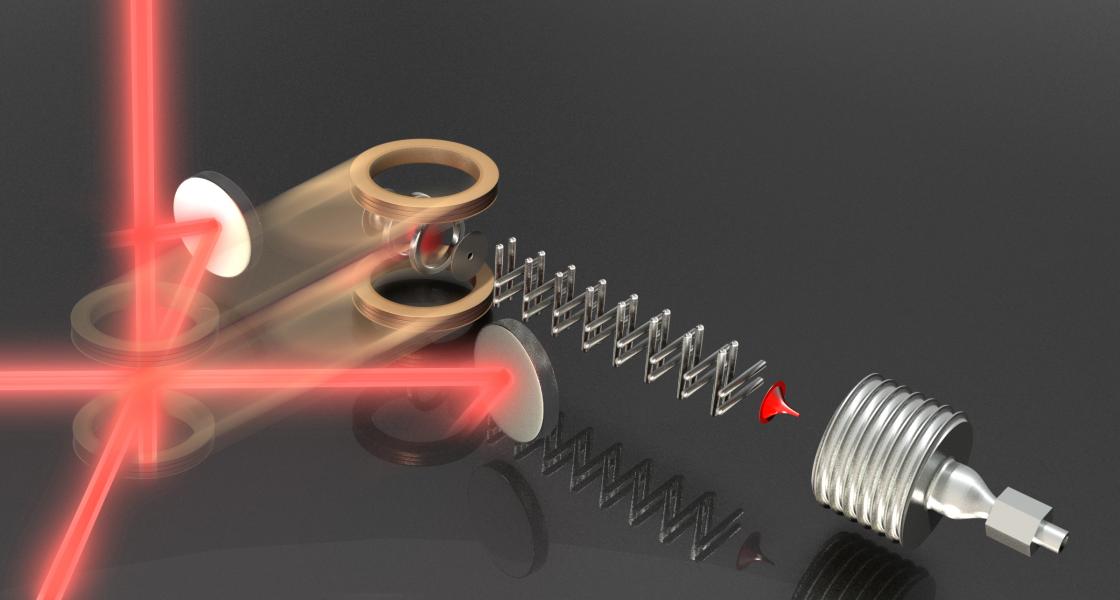
Their novel experimental setup is shown in the top picture (Figure 1). The researchers cool and trap Rb atoms at the intersection of the (red) laser-cooling beams. Then a pulsed valve (lower right) creates a beam of cold ND3 molecules. The metallic rods and rings create electric fields that slow and trap the molecular beam. To combine the cold molecules and ultracold atoms, the researchers physically move the coils forming the atom trap across the table until the atom trap overlays the molecule trap.
With the traps superimposed, atom-molecule collisions are likely to occur, according to theory. These collisions will have very little effect on an ND3 molecule. An ND3 molecule will usually remain in the same quantum state after a collision as it was in before anything happened.
To liven up their experiment, the researchers decided to see how electric fields would affect these unusual collisions. They quickly discovered that electric fields have a major effect on ultracold atom-cold molecule collisions. Even though electric fields affect only the orientations of the molecules, they increase the likelihood that a given atom-molecule collision will change the quantum state of the ND3 molecule. And, collisions occurred faster than expected.
The JILA researchers enlisted the help of theorist colleagues from the University of Durham (UK) to explain what was happening. New theory showed that electric fields strongly influence atom-molecule collisions — even if there are no dipole-dipole interactions (Figure 2). Dipole-dipole interactions occur between atoms or molecules that have slight differences in charge or magnetic field between one end and the other, resulting in an attraction between ends with opposite polarity. Such dipole-dipole interactions in ultracold quantum gases of potassium-rubidium (KRb) molecules have recently been a hot topic at JILA (See The Quantum Control Room)
However, an entirely different process is at work in the ultracold atom-cold molecule collisions studied by the Lewandowski group. In collisions that occur without an electric field, the pyramidal structure of the ND3 molecule is fairly stable. There is only a low probability that a collision will cause the pyramidal structure to flip inside out, i.e., change into a lower-energy quantum state. In contrast, when an electric field is present, the orientation of the ND3 molecule can get “confused” by competing forces that arise as an atom approaches. This confusion increases the probability of a state-changing collision. - Julie Phillips