In chemistry, the shape of a molecule matters. Different arrangements of the same molecule are called isomers, and scientists have spent decades pondering how that shape affects chemical reactions.
Molecules react, bond, and separate along a reaction pathway, twisting into different shapes and combinations before delivering their final products. Tracking that pathway, especially with two molecules that are otherwise identical, is extremely complicated for theorists and experimentalists alike—and leaves them with a lot of questions about the importance of the initial isomer’s shape.
“How does the structure influence reactions and the reaction pathway? If you have the same atoms put together in one way reacting with an ion versus another way, how does that change the pathway they go on, and ultimately the products that they produce?” asked JILA Fellow Heather Lewandowski.
To follow the paths of the isomers, the Lewandowski Group and their theory collaborators became detectives, looking for clues in the reactions between acetylene ions and propyne, and acetylene ions and allene. After years of careful study and theory calculations, their findings have been published in Physical Chemistry Chemical Physics—providing another piece of the puzzle in chemical reactions and the formation of the cosmos.
Heavy hydrogen tags
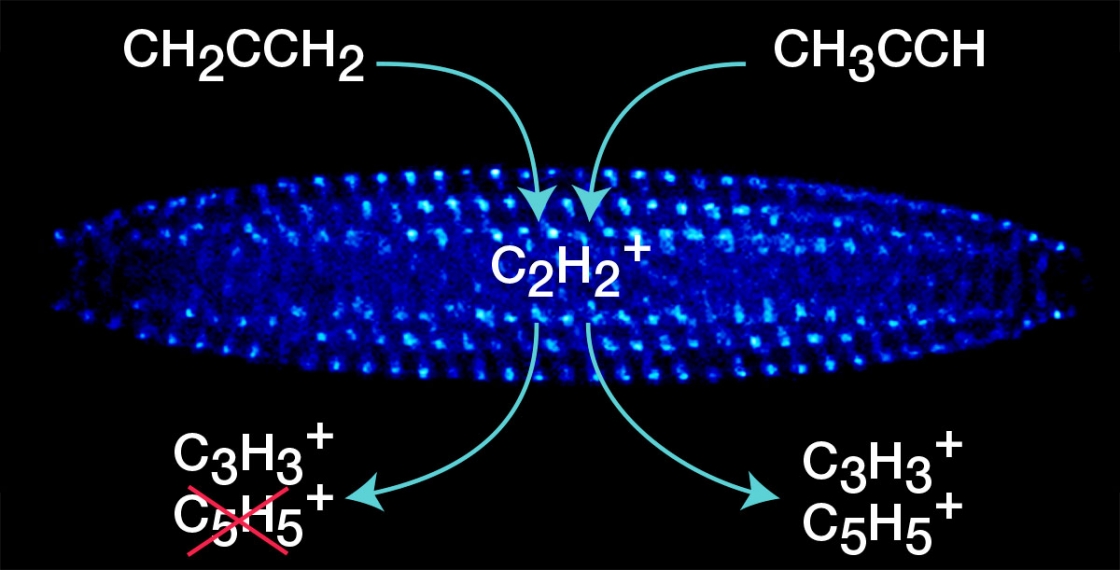
The group started with acetylene—the same gas you would find in a welding torch—and trapped it in an ion trap. Then they studied its reactions with two different isomers of C3H4—allene and propyne.
The acetylene and propyne or allene react, bonding and breaking apart. Then they open the trap and accelerate the ions, sucking them into an ion detector. Ions arrive at the detector at different times based on their mass—heavier ones later than the lighter ones.
Without any tools to directly probe the molecules along their reaction pathway, the Lewandowski Group worked closely with theorist John Stanton and his group at the University of Florida. To help track that pathway, the Lewandowski Group added deuterium—a “heavy” hydrogen atom—to their molecules. That heavier atom acts like a tag, adding a little extra mass to some of the final products. As the two molecules combine and break apart, the product with deuterium appears later on the ion detector.
If the deuterium ended up with one set of products, the theorists could calculate that the reaction took one pathway versus another. The Lewandowski Group painstakingly swapped deuterium into different combinations for the acetylene, allene and propyne to track all of the possible outcomes, and working with their theory collaborators to calculate what each outcome meant.
“We sort of do this piece by piece, and we gather all these data together to be able to make a self-consistent picture. It’s taken us several years,” Lewandowski explained. “This is really quite a complex reaction to understand at this level of detail.”
Eventually the team was able to clearly draw the reaction pathways from the acetylene and propyne, and the acetylene and allene combinations. Acetylene and allene only resulted in a single product, whereas there were many possible products from acetylene and propyne.
“Our question was if you have allene or propyne, do these react differently with acetylene just because they have this different structure? The answer is fundamentally yes,” Lewandowski said.
The electron fly-by
More importantly, these products revealed how these reactions start for these molecules: charge exchange. Lewandowski described charge exchange as an electron fly-by between the neutral molecule (the allene or propyne) and the molecule missing an electron (the acetylene).
“The idea is, for one of these reactions, the electron hops when these molecules are very far apart, in a relative sense,” she explained. In the allene-acetylene reaction, the electron can just hop on as the molecules speed past each other.
For propyne, the electron can’t hop on during a molecule fly-by. In order to hop, the molecules have to get close enough to crash.
“It hops, but they are already so close they come in and form a complex,” Lewandowski said, resulting in multiple outcomes. “The real difference between these two is that for one reaction they charge exchange when they are far apart and then the molecules keep on going, and therefore produce only one ion product. The other reaction trades that electron, and then falls together and sticks together and then reacts. That initial stage causes these two wildly different outcomes…The shape drives the reactions.”
That’s important information to understand how neutral and ionic molecules react. But these cold molecules also resemble how atoms in space might interact. These studies can help us understand how complex molecules form in space. However, there’s still a lot yet to learn, and many more puzzle pieces to gather before that picture is complete, Lewandowski added.
“We have one more piece in the puzzle in understanding how ions and neutral molecules react,” she said. “One of the ideas is to build on this with other ions and other neutral molecules to put this puzzle together.”
This study was done by an interdisciplinary group of physicists and chemists, including Philipp Schmid, James Greenberg, T.Lam Nguyen, James Thorpe, Kathrine Catani, Olivia Krohn, Kyle Miller, John Stanton, and Heather Lewandowski. It was published in Physical Chemistry Chemical Physics on August 31, 2020, and was supported by the National Science Foundation Physics Frontier Center grant, NIST, and the Air Force Office of Scientific Research.
Written by Rebecca Jacobson