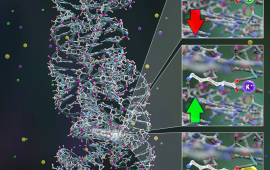
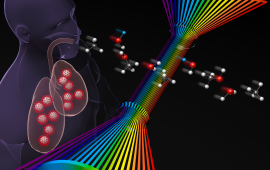
Chemical physicists at JILA use advanced laser techniques to probe the structure and dynamics of matter during chemical reactions, i.e., during the making and breaking of chemical bonds.
JILA’s chemical physics research includes studies of molecular energy flow and optical/electrical properties of crystals, mapping of electron dynamics in materials and individual molecules, fabrication of nanomaterials, cooling of molecules through Stark deceleration and laser manipulation, and identifying short-lived molecules within interstellar and atmospheric combustion. These research endeavors advance our understanding of chemical reactions and inform the development of new materials.