Research Highlights
Precision Measurement | Quantum Information Science & Technology
A New “Spin” on Ergodicity Breaking
Published: August 17, 2023
PI: Jun Ye | PI: David Nesbitt
Nanoscience
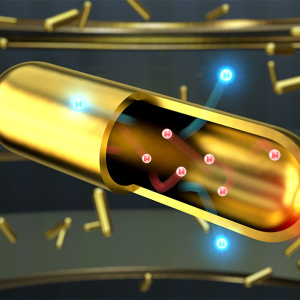
Going for Gold: New Advancements in Hot Carrier Science
Published: August 16, 2023
PI: David Nesbitt
Biophysics | Chemical Physics
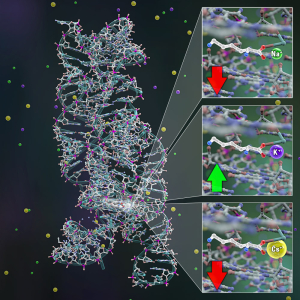
Looking at a Cellular Switch
Published: May 23, 2023
PI: David Nesbitt
Quantum Information Science & Technology
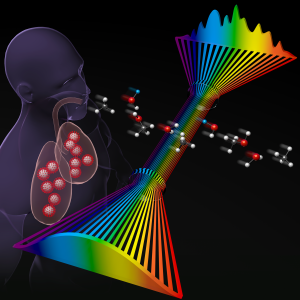
Using Frequency Comb Lasers as a Breathalyzer for COVID-19
Published: April 06, 2023
PI: David Nesbitt | PI: Jun Ye
Atomic & Molecular Physics | Biophysics | Chemical Physics
When Breath Becomes Data
Published: October 05, 2021
PI: Jun Ye | PI: David Nesbitt
Atomic & Molecular Physics | Chemical Physics | Precision Measurement
Overcoming Camera Blur
Published: August 10, 2021
PI: David Nesbitt
Laser Physics | Quantum Information Science & Technology
Guiding Electrons With Gold Nanostars
Published: March 13, 2020
PI: David Nesbitt
Chemical Physics
Way Faster than a Speeding Bullet
Published: June 18, 2012
PI: David Nesbitt
Biophysics | Chemical Physics | Nanoscience
It Takes Two to Tango
Published: April 12, 2009
PI: David Nesbitt
Biophysics | Chemical Physics | Nanoscience
Explosive Evidence
Published: February 27, 2009
PI: David Nesbitt
Biophysics | Chemical Physics | Nanoscience
Splash 2
Published: July 07, 2008
PI: David Nesbitt