Graduate student Erik Holmstrom and Fellow David Nesbitt have applied their laboratory research on the rates of RNA folding and unfolding to the medically important enzyme telomerase. Telomerase employs both protein and RNA components to lengthen chromosomes, which are shortened every time they are copied.
If one short piece of the RNA in telomerase is folded into an organized structure called a pseudoknot, then the enzyme works properly. The enzyme repeatedly adds short pieces of DNA to the chromosomes within the cells of people and many other organisms. Because it counteracts the natural shortening of chromosomes, telomerase is vital for keeping cells alive and healthy through multiple cell divisions.
If, however, the pseudoknot contains mutations that interfere with folding, the result can be the serious genetic disorder dyskeratosis congentia, which causes a variety of skin and blood diseases. New work by Holmstrom has revealed why mutations in the pseudoknot are so harmful.
When the pseudoknot unfolds, telomerase stops working. In normal people telomerase works efficiently because the pseudoknot is folded about 99.9% of the time. But, in people who suffer from dyskeratosis congentia, this enzyme does not work properly because it is only folded half the time. Holmstrom discovered why: In the laboratory, a mutated pseudoknot folds 400 times more slowly than a normal pseudoknot and unfolds five times faster.
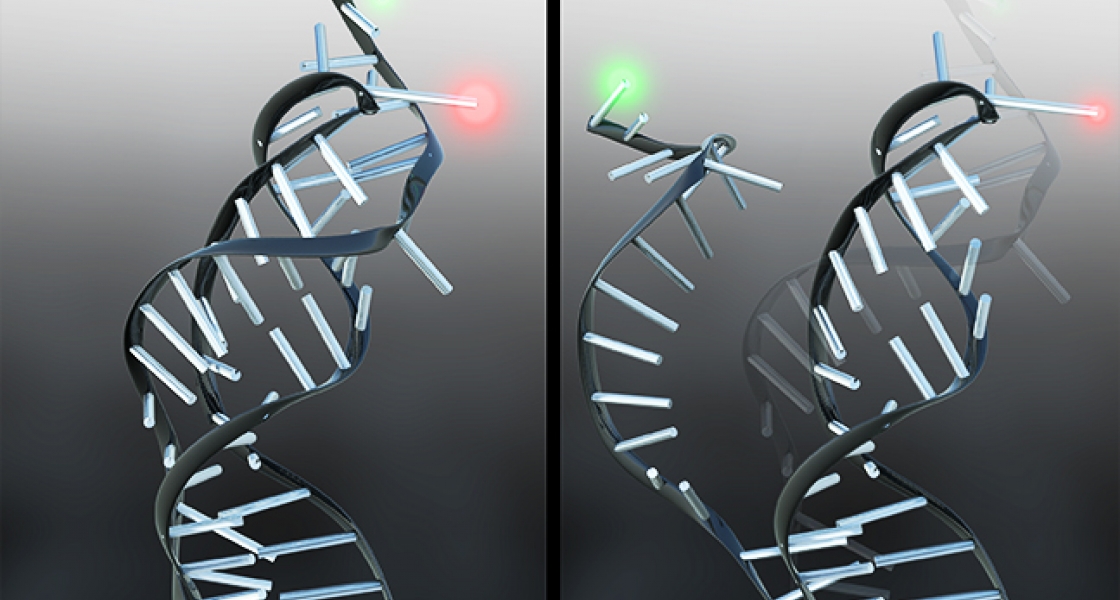
Holmstrom was able to determine the rates of folding and unfolding using a technique known as single-molecule fluorescence resonance energy transfer (smFRET). He attached dyes that fluoresce green and red to a small piece of RNA consisting of just the pseudoknot. Then Holmstrom shined laser light on the pseudoknots, causing the green dye to to fluoresce green. When the RNA folds, the two dye molecules are brought close together, which allows the green dye to transfer energy to the nearby red dye, causing it to fluoresce red. By carefully monitoring the patterns of red and green dots in a microscope, Holstrom could “watch” the pseudoknots fold and unfold, which provides information about the rates at which these processes were occurring.
The new understanding of the role of the pseudoknot in regulating telomerase activity may one day lead to improvements in two important areas of medicine: tissue regeneration and cancer treatment. For successful tissue regeneration from stem cells, medical researchers will benefit from being able turn on and maintain telomerase activity until a new liver or pancreas is built up cell by cell. On the other hand, oncologists want to develop new drug therapies to turn off active telomerase, which is present in 85–90% of all cancerous tumors. If telomerase were disabled by drugs, cancer cells would stop dividing as soon as their chromosomes lost enough DNA to irreversibly damage their genetic code.
This work is a good example of how basic research in biophysics may lead to future medical advances.—Julie Phillips