Research
Updated on June 7, 2013
Solvation effects on the chemistry of objects from
molecules to nanocrystals
Much of the behavior of important molecular species is
only known in a condensed phase environment (mostly
solutions), where interaction with the solvent changes
some of the properties of the solute. In order to
unravel the effects of solvent-solute interactions, we
want to understand the intrinsic
properties of the solute and study them as isolated
entities in vacuo. There are several areas of
interest at present:
(A) Understanding reaction mechanisms in
water oxidation catalysis:
One of the most promising avenues towards a
sustainable energy economy, independent of the use of
fossil fuels, is the conversion of CO2 and
water into chemical fuels. For this process to be
economically viable, catalysts need to be developed for
the various steps involved, but the molecular-level
mechanisms in the catalytic generation of solar fuels
are often poorly understood. At the current stage of
research, it is important to investigate promising
molecular catalysts and key intermediates in the
proposed catalytic cycles in detail to connect molecular
properties with catalyst performance, which can
potentially guide catalyst design. We use electrospray
ionization to bring water oxidation catalysts into the
gas phase, where we can characterize their intrinsic
properties with electronic and vibrational spectroscopy.
We can also manipulate them by preparing clusters with a
well-defined number of solvent molecules, building the
solvation environment one molecule at a time, and
following the evolution of their properties with
increasing solvation. You can click here
to read more.
(B) Reductive activation of CO2 by
transition metal catalysts:
Image
Credit: Steven Burrows
Water oxidation (see above) is only side of the coin,
the
other is CO2 reduction. 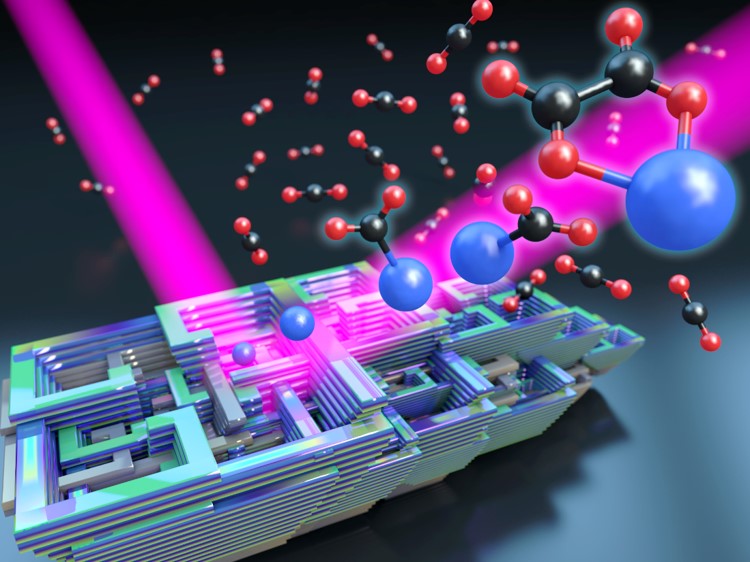 So
far, mechanisms and especially solvent effects in CO2
reduction catalysis are largely not understood or even
characterized. Mass-selected clusters of metal anions
with CO2 serve as model systems for the
reductive activation of CO2 by a catalyst
under complete control of the composition and size of
the solvation environment. Vibrational
spectroscopy and electronic structure calculations are
used to obtain molecular-level information on the
interaction of solvent with the catalyst-CO2
complex and their effects on one-electron reduction of
CO2. If you would like to read more about
this, click here
and here.
So
far, mechanisms and especially solvent effects in CO2
reduction catalysis are largely not understood or even
characterized. Mass-selected clusters of metal anions
with CO2 serve as model systems for the
reductive activation of CO2 by a catalyst
under complete control of the composition and size of
the solvation environment. Vibrational
spectroscopy and electronic structure calculations are
used to obtain molecular-level information on the
interaction of solvent with the catalyst-CO2
complex and their effects on one-electron reduction of
CO2. If you would like to read more about
this, click here
and here.
(C) Photochemistry and electronic structure of
complex ions:
Image Credit: Steven
Burrows
We gain a deeper insight into the electronic and
geometric
structures, and the inter- and intramolecular forces in
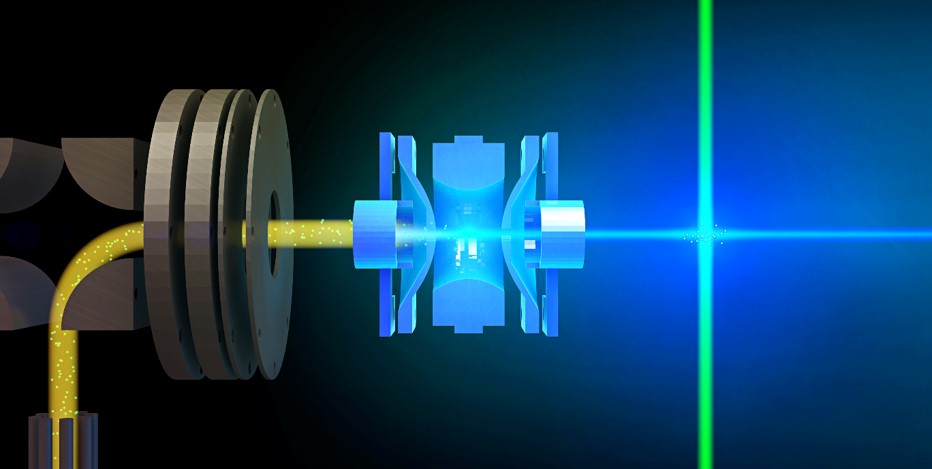 complex ions. The experiments contribute
valuable information e.g. on the electron
donation/back-donation in metal- and metaloxide-ligand
complexes and electron binding energies. Another
example in this program area is the investigation of the
photochemistry of species that are relevant to
metal-organic reactions, e.g. the photochemistry of
chromate esters, which are important intermediates in
the oxidation of alcohols by chromate. If you are
interested in this area, you can read more here,
here,
and here.
We use
complex ions. The experiments contribute
valuable information e.g. on the electron
donation/back-donation in metal- and metaloxide-ligand
complexes and electron binding energies. Another
example in this program area is the investigation of the
photochemistry of species that are relevant to
metal-organic reactions, e.g. the photochemistry of
chromate esters, which are important intermediates in
the oxidation of alcohols by chromate. If you are
interested in this area, you can read more here,
here,
and here.
We use
cryogenic ion spectroscopy of mass selected ions in this
work.
In this project, we are developing methods for the preparation and spectroscopic study of nanocrystals suspended in a buffer gas under well-controlled experimental conditions. The main tool set in this project consists of cryogenic buffer gas beam and trap techniques. These techniques have been developed in AMO physics and physical chemistry communities, but have been mostly used on relatively small neutral molecules (in the case of cryogenic buffer gas beam sources) and somewhat larger ions (in the case of cryogenic ion traps). We want to use the best parts from both worlds to develop an apparatus that will be capable of preparing nanoparticles under cryogenic conditions. These can then be studied using mass spectrometry and fluorescence spectroscopy. The aim of the project is to enable fundamental studies of the intrinsic properties of nanocrystals under well-controlled conditions, which means in particular the absence of any chemical environment such as solvents, matrices, or surfaces.
Supramolecular chemistry and materials at very high pressures
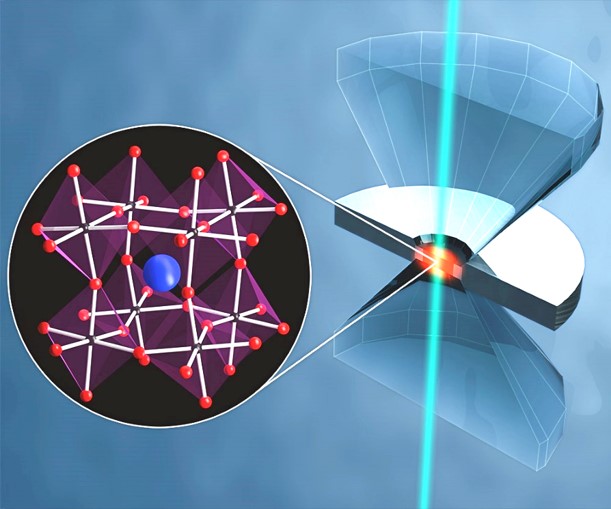
Nanostructured materials (quantum dots, nanowires,
nanocrystals) have led to a large research field in the
last years. While there is a huge body of work on their
synthesis, the molecular-level details of their
interaction with their chemical environment is largely
not explored, as is the size dependence of much of their
structural properties. High pressure experiments offer
access to tackle such questions.
At relatively low pressures (a few
hundred MPa), only intermolecular distances are varied
by application of pressure. This is much less
perturbative than variation of temperature or chemical
composition. The behavior of nanocrystals at these
low
Image Credit:
Steven Burrows
pressures yields information on the properties of the
interface
between thenanocrystals and
their chemical environment, including their protective
ligands. Higher pressures (GPa range) can result in
phase changes in the solvent or in the nanocrystals
themselves. The former will again lead to a change in
the interaction of the nanocrystal with its environment.
The latter afford access to new materials with new
optical and electronic properties. We use
photoluminescence spectroscopy and Raman
microspectroscopy to study these phenomena. You can see
examples of our work here
and here.



