Spectroscopic Studies of Ionic Transition Metal Complexes
Highlights
Jesse C. Marcum and Sydney H. Kaufman, two graduate students in the Weber group, have characterized the photochemistry of the gold salt tetrachloroaurate, Au(III)Cl4-, and its dihydroxylated analog, Au(III)Cl2(OH)2- in the gas phase. Gold salts are important species in a number of chemical areas. In geochemistry, these gold salts represent ways of how gold is transported in water. Moreover (and perhaps more importantly), Au(III)Cl4- is the most important precursor for the production of gold nanoparticles and can be reacted to other gold salts replacing chlorine atoms by hydrody groups (OH)
(e.g. leading to Au(III)Cl2(OH)2-) by decreasing the acidity of the solution. Metal nanoparticles hold enormous promise in applications ranging from drug delivery to new materials with unusual optical properties. Most of the current synthetic routes use wet chemistry to achieve nanoparticle formation. Photochemical approaches may offer additional control over the nanoparticle synthesis by choosing the best possible wavelengths and by achieving spatial control over where the reaction takes place in solution. In order to obtain information on the intrinsic properties of the salt and the influence of the solvent, it is important to characterize the photochemistry of the process both in solution and in vacuum (i.e. in the absence of solvent).
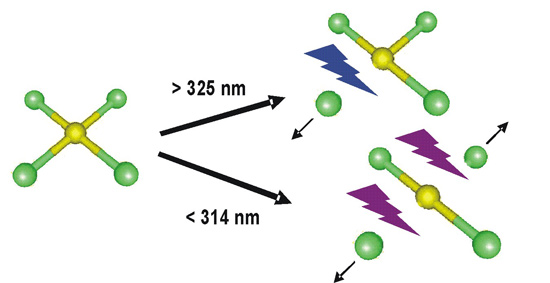
The Weber group has studied the photofragmentation of
AuCl4- and Au(III)Cl2(OH)2-
in the absence of solvent. They found that the
fragmentation of AuCl4- in vacuum
is highly wavelength-specific. Wavelengths longer than
325 nm result at best in the loss of one chlorine atom,
wavelengths shorter than 314 nm result in the loss of
two chlorine atoms. the former process leaves behind
AuCl3- as a product, while the
latter process generates AuCl2-.
Both are intermediates along the route to producing gold
nanoparticles. Utilizing wavelength specific reactions
may offer the prospect to tune the photochemistry of the
salt by choosing the laser wavelength for the light
employed in this process. At the same time, the new data
show at which wavelengths AuCl4-
absorbs ultraviolet light in vacuum. Comparing the
absorption wavelengths from experiments under vacuum
with those in solution may lead to similar control over
the photochemistry of this gold salt in solution and
therefore new routes to better control in the production
of gold nanoparticles.

In addition, the researchers found that AuCl4-
fragments by losing one or two ligands, while AuCl2(OH)2-
always loses two of the ligands. This may shed light on
a little-used synthetic approach in this area, namely a
combination of making the solution less acidic and using
UV light to activate the gold salt used. This could lead
to a new route in nanparticle synthesis.



