Former research associate Antonio Picón, research associate Agnieszka Jaron-Becker, and Fellow Andreas Becker have discovered a way to make the hydrogen molecular ion (H2+) fall apart into its constituent atoms without exciting or ionizing the electron. This startling finding was a big surprise for the researchers, who recently figured out how to do something that conventional wisdom said was difficult, if not downright impossible.
For starters, molecules usually don’t split up into atoms unless at least one electron is excited or ionized. Rather, they dissociate into charged ions, with one negatively charged ion containing one or more extra electrons and another positively charged ion missing the same number of electrons.
Researchers thought that the only two ways to break apart a molecule into neutral atoms would be (1) to hit it with a rapid sequence of laser pulses over a wide range of different frequencies (i.e., energies) or (2) to change the frequency of the laser pulse over time, a process called chirping. Either way, the goal was to get the molecule shaking hard enough to fall apart. Either process is technically challenging since the laser frequencies need to exactly resonate with the molecule’s vibrational states to make it fall apart. And, these vibrational states get closer and closer together as they get higher and higher in energy. And, as the vibrational states get closer together, they are harder to stimulate with a single laser pulse.
Two years ago, Agnieszka Jaron-Becker and Andreas Becker weren’t even thinking about getting molecules to fall apart. Rather, they were exploring the behavior of atoms and molecules interacting with infrared (IR) lasers. Their research was related to the new IR laser used to produce coherent high-energy soft x-rays in the Kapteyn/Murnane (K/M) group. They found little prior work on IR lasers because the response of matter to lower-energy light was considered less exciting than its response to visible and higher-energy wavelengths of light.
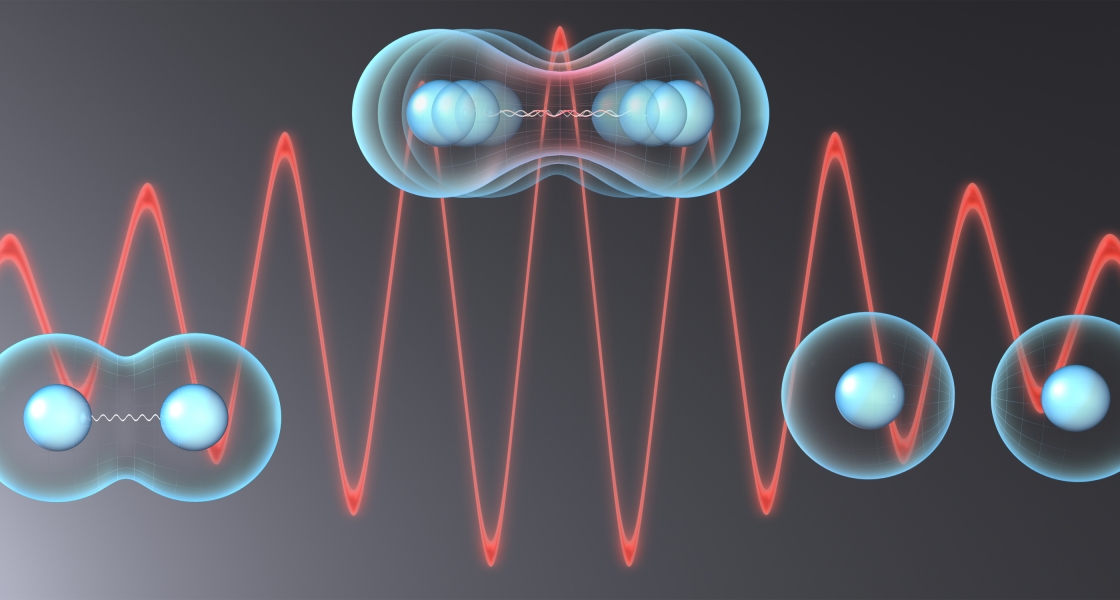
Near the end of 2010, working with Picon, Jaron-Becker and Becker discovered that IR laser pulses would greatly enhance vibrations of simple molecules such as H2+. They showed that when this molecule absorbs two photons of IR light, the photons excite the molecule’s higher vibrational states.
Then, in an exciting new study just published in Physical Review Letters, the researchers showed that the absorption of pairs of photons of IR light of the right wavelength strongly favors the dissociation of molecules into atoms. A range of wavelengths is not needed! In theory, pulses of 12-µm IR light will excite all the vibrational states of H2+ at the same time, causing the molecule to rapidly dissociate into its constituent atoms. The molecule shakes itself apart rather than ionizing!
Unfortunately, there are not yet many IR lasers with a wavelength as long as 12 µm available to test the new theory. Consequently, the researchers are looking for a simple nonpolar molecule whose vibrational states will all resonate from the absorption of two photons of light from the 4-µm IR laser already used by the K/M group. Andreas Becker hopes it’s only a matter of time until they find one.
Stay tuned for more exciting developments in the physics of molecule dissociation!