If you want to control the quantum world, it helps to make things really cold—like a few millionths of a degree above absolute zero. When atoms reach those ultracold temperatures, they slow down and scientists can better probe them and study their interactions.
Ultracold atoms have been well-explored for decades, and are the basis for precision metrology tools like atomic clocks. With dense collections of ultracold atoms, physicists have been able to study how atoms interact, leading to new understanding of strange quantum mechanical phenomena.
Yet, atoms are fairly simple—a nucleus with orbiting clouds of electrons. So what could we learn from more complex systems like molecules?
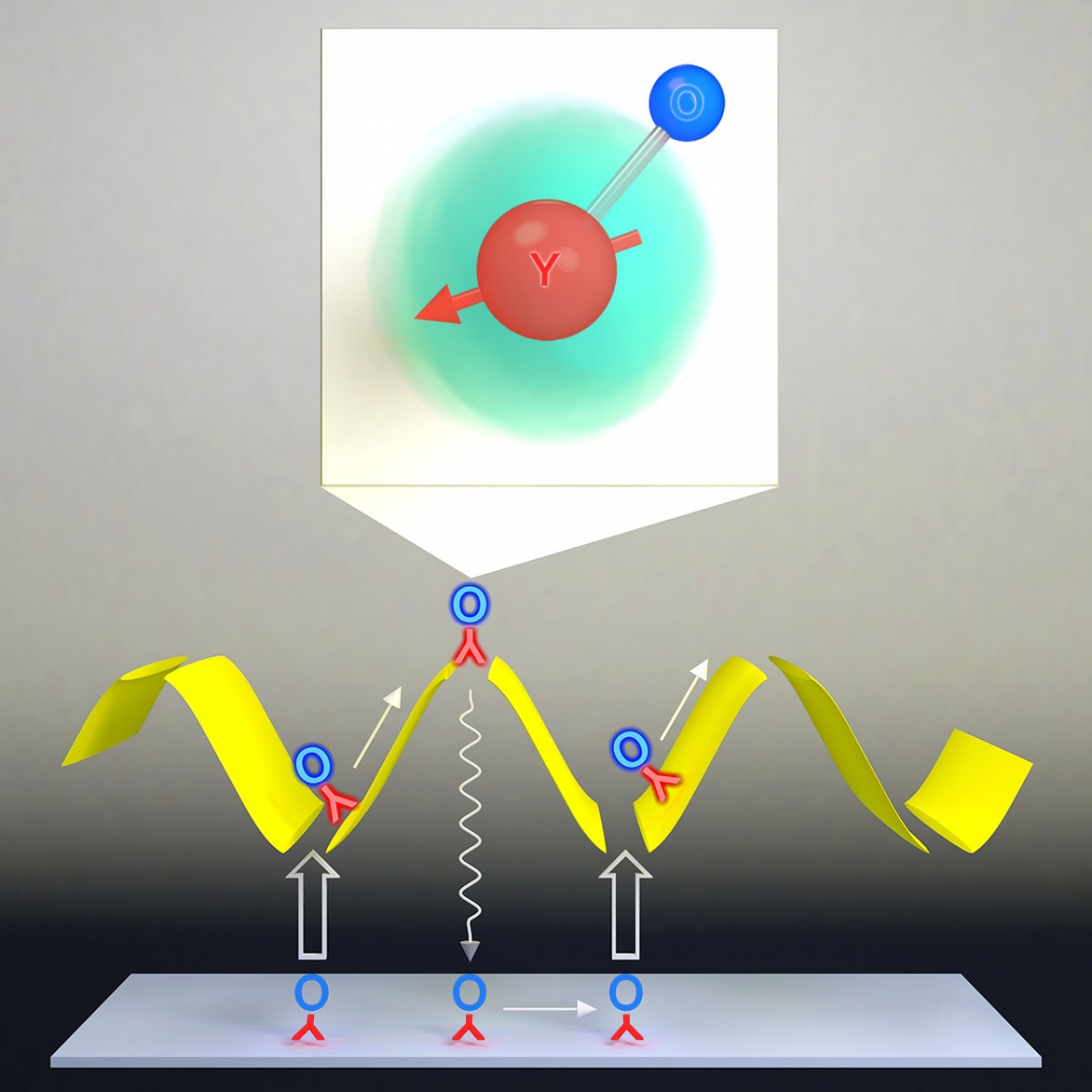
“Molecules have very different properties,” said Shiqian Ding, a postdoctoral researcher in the Ye Lab. Compared to atoms, molecules have more degrees of freedom and richer energy structures, which create opportunities to see new quantum mechanical phenomena, understand how chemistry works on the most fundamental level, and develop new technology platforms for quantum control. However, that complexity also makes it more challenging to extend laser cooling techniques—which work so well with atoms—to molecules.
Techniques exist to bring a pair of cold atoms together to form a molecule, but laser cooling molecules can bring a more diverse set of molecules to ultra-low temperatures. Using a technique called gray molasses cooling, Ding and his team—Yewei Wu, Ian Finneran, Justin Burau, and JILA Fellow Jun Ye—have cooled yttrium monoxide molecules to 4 microKelvin—4 millionths of a degree above absolute zero.
They found that this technique is not only efficient, but also robust, keeping molecules cold even under unusual circumstances, such as in the presence of a very large magnetic field. They also proposed and demonstrated a novel scheme to combine this robustness with a trapping force to increase the molecular density by a factor of 10. Their findings were published in Physical Review X on June 3.