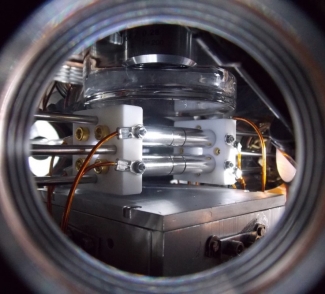
Ion traps afford an exquisite level of control for charged atoms and molecules. Our lab directly laser cools trapped calcium ions (Ca+) down to millikelvin temperatures for the purpose of studying gas-phase cold chemical reactions. The balance of trapping forces and Coulomb repulsion cause the trapped, cryogenic ions to arrange themselves into quasi-periodic structures called Coulomb crystals. The cold conditions of the trap allow us to explore fundamental reaction mechanisms as well as gas phase chemistry relevant to extreme environments (e.g., interstellar medium, planetary atmospheres, and combustion).
Topics