5. THE DOPPLER EFFECT:
As we have discussed, atoms and molecules emit and absorb radiation at particular frequencies that are seen as spectral lines once the radiation is dispersed through a spectrometer. But the frequency (or wavelength) that is observed is not the same as the frequency that is emitted or absorbed if the atom is moving away from us or toward us (likewise, if we are moving away from the atom or toward it). This change in frequency is called the Doppler Effect. If the observer and the source are moving apart, the observed frequency decreases: the shift in frequency is called a red shift. If the observer and source are moving closer together, the observed frequency increases: the shift is called a blue shift. The Doppler effect occurs for all kinds of waves, including sound waves, water waves, and all bands of electromagnetic radiation.
The idea is illustrated here: Doppler Physlet. For electromagnetic radiation, you should select the option marked "relativistic", then hit the run button. First count as wave crests leave the source (the source frequency). Then place your cursor in front of the source and count as wave crests pass the cursor. You will see that your count rate (frequency) is higher than the source frequency: a blue shift. Then repeat the experiment with the cursor behind the source to see a red shift.
If the source is moving sideways with respect to the observer, getting neither closer nor further, the Doppler shift is negligible.* *Actually, there is a slight redshift if the velocities are very high, due to a phenomenon called time dilation that comes from Einstein's theory of relativity. We will discuss that later.
Measuring the frequency shifts of spectral lines is a powerful tool for measuring the velocities of objects. If you doubt that, try speeding on Regent Drive. You will soon meet a policeman there and he will be happy to show you his Doppler radar gun. It emits microwave radiation at a particular frequency. The radiation bounces off your car and is reflected to his gun, which measures the received frequency with high accuracy. If you are moving toward the policeman, the reflected radiation has higher frequency (a blue shift), and if you are moving away, lower frequency (a red shift). This experiment will cost you $50 or more, depending on the measured frequency shift (which tells your speed).
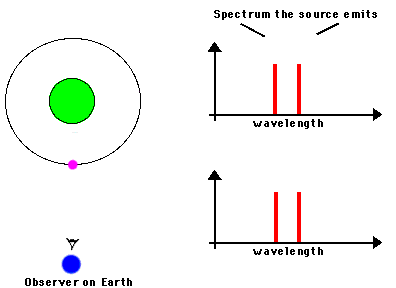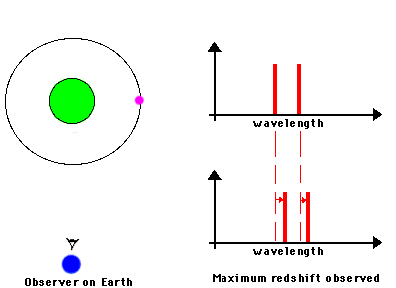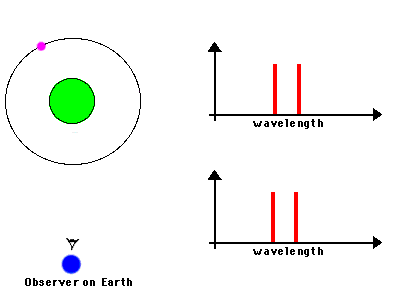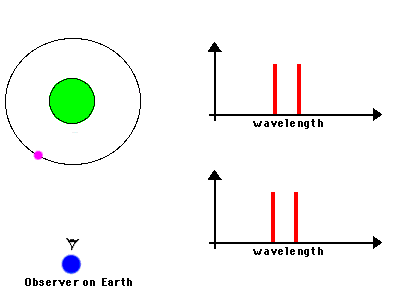
Likewise, astronomers can measure the speed of an astronomical source by observing the frequency shifts of spectral lines from atoms or molecules in the source. We know the frequencies at which a particular atom emits radiation because we can measure these frequencies from the same atom in laboratory experiments. If the observed frequency is shifted toward the blue (or red), we can attribute that shift to the fact that the atoms in the astronomical source are moving toward (or away) from us. Here's a nice illustration of how such shifts can be used to measure the orbital motions of stars in binary systems:

Source: NASA's Imagine.
In Chapter 9, you will see how this technique has been used to find planets around nearby stars. Toward the end of this course, you will learn how astronomers proved that the universe is expanding from an explosion that occurred some 12 billion years ago. That was accomplished through measurements of the Doppler shifts of spectral lines.
We can also use the Doppler effect to measure the temperature of an astronomical source from the widths of spectral lines. Atoms in a gas are always moving in random directions. The average speed of these motions defines the temperature of the gas. If the gas is hot, the average speed is higher. Some atoms in the gas will be moving toward us, and some will be moving away from us. We see radiation emitted by the atoms moving toward us at frequencies that are slightly blue-shifted, and radiation from the atoms moving away from us at frequencies slightly redshifted. As a result, the spectral line that we see from the combined emission from all of these moving atoms is broader than it would be if all the atoms were at rest, and the width of the line is a measure of the temperature of the gas.
(Return to course home page) Last modified January 18, 2002;
Copyright by Richard McCray