One way to understand unstable molecules is to systematically create slightly different versions of a similar stable molecule and investigate each new molecule with identical analysis and experiments. That is exactly what researchers from JILA and CU are doing with a series of ringed molecules. The JILA researchers are Graduate Student Adam Gianola, Postdoctoral Research Associate Takatoshi Ichino, and Fellow Carl Lineberger. Their CU collaborators are Lecturer Rebecca Hoenigman, Senior Research Associate Shuji Kato, and Professor Veronica Bierbaum.
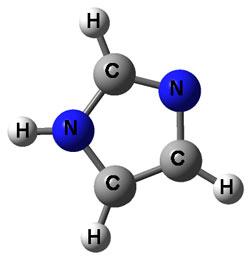
The investigators began their work with a ring of 5 carbons (C), each with hydrogens (H) attached to it (C5H6). Then, they evaluated a ring with a nitrogen (N) atom substituted for one of the C-H groups in the ring, creating pyrrole (C4H5N). Next, they substituted a second N atom into the ring, creating imidazole (C3H4N2). By substituting additional N atoms into the ring in a stepwise fashion, they eventually hope to study a 5-member ring made of all N atoms, pentazole (N5H). This latter structure's properties are so difficult to measure that no one has ever succeeded in doing it.
Gianola and his colleagues are using a two-step process to investigate the series of ringed molecules. First, they use hydroxide (OH-) to remove protons (H+) from the N atoms, leaving the molecules as negative ions. Second, they bombard the negative ions with a laser photon beam, removing the extra electrons from the ions and returning the molecules to a neutral-charge state. The experimenters collect the photodetached electrons and measure their energy distribution. These measurements provide information about the resulting neutral molecules, which are unstable.
The researchers also measure the energy changes that occur when the proton is removed in step 1. As shown at left, it is not only possible to remove a proton from the N atom, but also from a C atom. The energy distribution of electrons removed in step 2 allows the researchers to determine which protons were removed.
Thus far, it appears that as you add more N atoms to the ring, it gets easier to remove a proton from one of them in step 1 but harder to remove the extra electron in step 2. The researchers are currently investigating a ringed molecule with three N atoms. They are working with Postdoctoral Research Associate Jeff Rathbone and Graduate Students Django Andrews and Ryan Calvi.
The investigation of the pyrrole molecule appeared in the November 18, 2004, issue of the Journal of Physical Chemistry A. The study of the imidazole molecule was published in the December 22, 2005, issue of the Journal of Physical Chemistry A. - Julie Phillips