JILA’s quest to determine whether the electron has an electric dipole moment (eEDM) began in 2006 with a suggestion by Fellow Eric Cornell that the molecular ion hafnium fluoride (HfF+) might be well suited for an eEDM experiment. An electric dipole moment is a measure of the separation of positive and negative charges in a system. If an electron does have an electric dipole moment, it’s a pretty darn small one. So small, in fact, that if the electron were the size of the Earth, its eEDM would only alter the planet’s roundness by less than the width of a human hair. But, even a very small eEDM would have large implications for our understanding of fundamental physics. Consequently, the John Bohn group did a theoretical study of HfF+ and found Cornell’s intuition was right on the money. (See JILA Light & Matter, Fall 2006). The Cornell group soon began preparing to meet the challenge of detecting it in the laboratory.
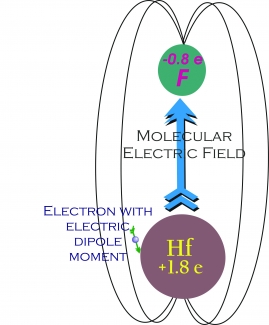
Five years laterthe Cornell and Ye groups are collaborating on an experiment to detect an eEDM — if it exists. The groups are investigating HfF+ because the two atoms in this molecular ion are capable of creating a huge internal electric field in response to a much smaller field applied to it in the laboratory. A small electric field applied in the laboratory to an ensemble of trapped HfF+ ions would precisely align all the internal electric fields of the ions, making it possible for researchers to test for an eEDM in an unpaired electron located near the Hf atom, as shown in the drawing.
But, before the scientists can attempt to identify an eEDM signal, they must understand the electronic structure of HfF+. That’s they only way they can identify which tree in a whole forest of normal transitions in the ion is most suitable for observing the coveted eEDM signal.
Unfortunately, researchers have only recently become interested in HfF+, and there is as yet little experimental data on its electronic structure. Even theoretical models have large uncertainties. This situation is about to change, however, thanks to graduate students Laura Sinclair and Kevin Cossel, undergraduate Tyler Coffey, and Fellows Jun Ye and Eric Cornell.
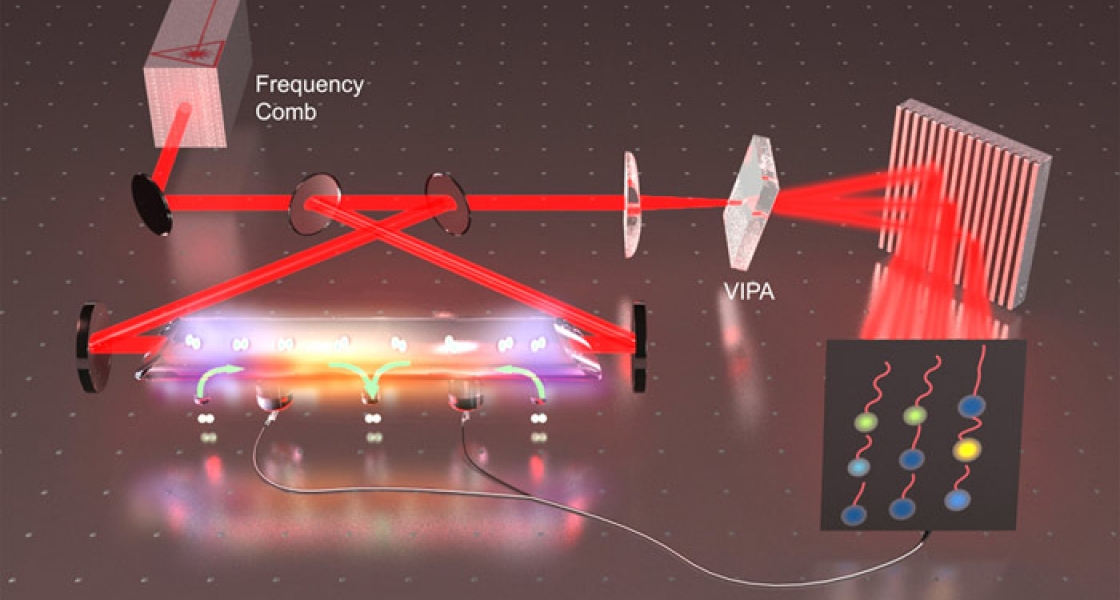
Sinclair and her colleagues recently invented an ultrahigh-sensitivity, high-resolution technique called frequency comb velocity-modulation spectroscopy that will make it possible to rapidly identify and characterize the electronic structure of HfF+. In a nutshell, the new technique shakes up a mixture of HfF+ ions and HfF molecules. Shaking modulates just the signals coming from the ions. The researchers then hone in on the modulated signals by looking for either increases or decreases in frequency. It’s like pinpointing the location of an ambulance running with a siren by analyzing whether the sound you hear is getting louder and higher pitched (as it comes closer to you) or softer and lower pitched (as it moves away from you).
This powerful and efficient new technique allows the researchers to rapidly look for many signals at once. In only 30 minutes, the researchers can precisely identify many transition frequencies over a wide range by finding the relatively small frequency shifts set off by the shaking. Without the technique, it would take the researchers at least two weeks to painstakingly gather the same information. It’s as if there were many ambulances scattered around a city and you wanted to find them all, you would have two choices: (1) you could go to every street corner and listen for the sirens, or (2) you could place microphones on the street corners and listen to all of them at once.
Sinclair, Cossel and their colleagues have invented a technique that essentially allows them to quickly see which “microphones” are picking up a signal and then scan those locations for multiple different transition frequencies. Their goal is to identify the electronic transition expected to be most conducive to revealing an eEDM during their upcoming experiment.
To finish laying the necessary groundwork for the experiment, Cossel is now leading the team working on characterizing HfF+. But, just to be sure he and his colleagues have the best possible chance for success, they are also using frequency comb velocity-modulation spectroscopy to probe the electronic transitions of radioactive thorium fluoride (ThF+), which may be an even better candidate for an eEDM experiment. ThF+ lasts longer before decaying than HfF+. It is also predicted to be approximately three times more sensitive to the presence of an eEDM than HfF+. Now that the researchers have such a powerful tool for probing the electronic structure of a molecular ion, they’ll soon be able to determine whether ThF+ or HfF+ stands the best chance of producing a measurable signal from an eEDM.