Fellow Tom Perkins’ group is significantly closer to realizing its long-standing dream of using atomic force microscopy (AFM) to study how membrane proteins fold and unfold. Historically, scientists have used AFM to measure the mechanical forces needed to unfold individual proteins and the resulting increase in their lengths. However, the limitations of AFM itself have prevented researchers from watching the unfolding process in detail.
For AFM to resolve protein unfolding in detail, three things must happen: (1) AFM must respond rapidly enough to shifts in protein conformation to detect short-lived folding-related changes, (2) its force measurements must be precise, and (3) the force measurements must be stable over time. Unfortunately, with commercial AFM cantilevers (the delicate diving-board-like structures to which the measurement tip is attached), researchers could only get one or two of these things to happen at the same time, but never all three.
That’s why the Perkins group has just spent more than five years improving AFM cantilevers and tips, which are actually attached to the protein under study. Most recently, the researchers modified a short AFM cantilever to be much less noisy and much more stable. These two changes allowed them to resolve the motion of single proteins! This long-sought capability was made possible by the merger of nanofabrication and biophysics. It was reported online in ACS Nano in March of 2014.
CU undergraduate Matt Bull (now a graduate student at Stanford University) led the research effort. The goal was to make flexible, but short, cantilevers. However, like a shorter diving board, shorter cantilevers are inherently stiffer. Bull used (and improved upon) a popular nanofabrication technique to come up with a soft and short cantilever capable of making precise force measurements. And, he did this without sacrificing long-term stability or the ability to detect changes in real time.
The three key modifications of the short AFM cantilever were (1) the use of a focused ion beam to carefully cut out a large rectangular hole at the base of the cantilever, increasing its sensitivity and responsiveness; (2) the addition of a transparent protective patch over the cantilever’s gold coating, preserving the cantilever’s high reflectivity; and (3) the removal of the remaining-unprotected gold coating from cantilever and the probe tip, enhancing stability. These improvements resulted in dramatically improved cantilever performance.
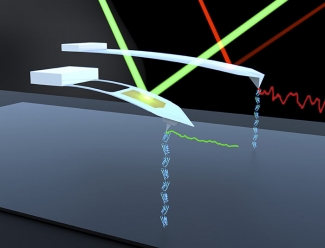
To show that the new cantilevers worked with real proteins, Bull and his colleagues mechanically unfolded, then studied a collection of proteins connected head to toe. The new enhanced cantilevers performed well, as shown by the green signal in the picture. The relatively noisy red signal came from the group’s previous favorite cantilever. The new cantilevers were 50 times better at detecting protein motions in real time, with no loss of stability. The enhanced stability was even preserved when the researchers pulled on a protein anchored to a surface.
After years of effort, the Perkins group has an AFM setup that leads the world in force precision and force stability. It is poised to watch individual proteins fold and unfold in minutes, a feat that is a 25-fold improvement over what was possible before.
What’s more, the new cantilevers are easy to make, easy to use, and relatively inexpensive. Some group members have already begun investigations of the folding and unfolding of membrane proteins with the new homemade AFM cantilevers. They’re focusing on membrane proteins because these proteins are the targets for 50% of all drugs, making them of great interest to medical science.
Other group members continue to work on improving AFM technology to make it an even better tool for research on single biomolecules. Better instruments that can track folding and unfolding over seconds rather than minutes will allow researchers to probe deeper into the complex behavior of molecules that make up living organisms. —Julie Phillips and Tom Perkins